AstraZeneca Pulls COVID-19 Vaccine, Citing ‘Surplus Availability’
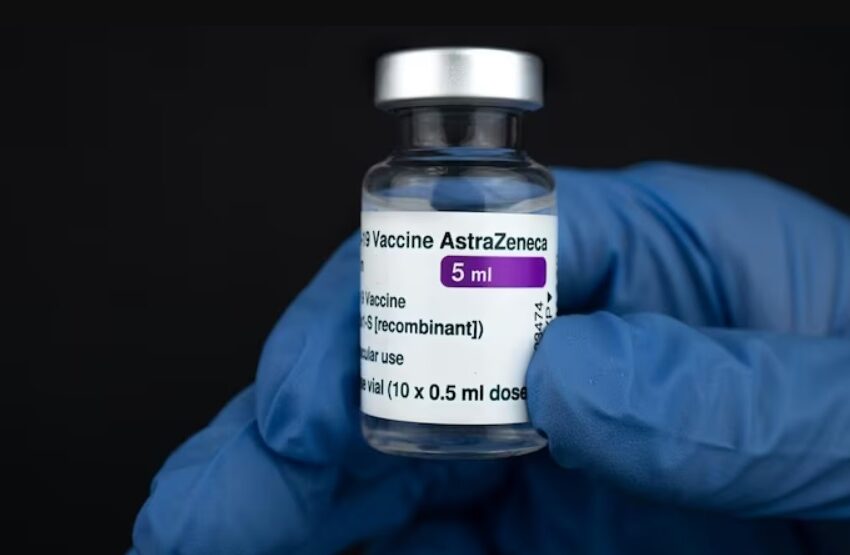
UK-based pharmaceutical major AstraZeneca has started global withdrawal of its Covid-19 vaccine, which was provided in India as ‘Covishield’ in partnership with Serum Institute of India, days after it admitted to rare side effects of blood clotting and low platelet counts.
The withdrawal has been initiated due to a surplus of available updated vaccines since the pandemic, the company said in a statement.
AstraZeneca had partnered with Oxford University to develop the Covid-19 vaccine, which was sold in India as Covishield and as Vaxzevria in Europe.
“As multiple, variant Covid-19 vaccines have since been developed there is a surplus of available updated vaccines. This has led to a decline in demand for Vaxzervria, which is no longer being manufactured or supplied,” it said.

The company further said, “We will now work with regulators and our partners to align on a clear path forward to conclude this chapter and significant contribution to the Covid-19 pandemic.”
Earlier, according to global media reports, AstraZeneca had admitted that its Covid-19 vaccine could in very rare cases has the potential to cause a rare side effect called — Thrombosis with Thrombocytopenia Syndrome (TTS).
Without referring to the side effects, the company said, “we are incredibly proud of the role Vaxzevria played in ending the global pandemic. According to independent estimates, over 6.5 million lives were saved in the first year of use alone and over three billion doses were supplied globally.”
It further said, “our efforts have been recognised by governments around the world and are widely regarded as being a critical component of ending the global pandemic.”
In India, over 2.2 billion dosages of Covid-19 vaccines have been administered and a majority of those were Covishield.